Is chain waxing worth the time and expense?
#101
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,342
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6201 Post(s)
Liked 4,204 Times
in
2,358 Posts
I didn't say the whole chain gets warm. The pivot actually carrying the load warms momentarily because it is actually a very small surface under a lot of strain as the link straightens off the cassette or bends around the chainring under load.
This effect is familiar to some knife sharpening people who are aware that dry honing has the effect of raising the temperature at the very edge momentarily high enough to affect the temper, leading to rolled edges. So I don't think it is far fetched to think that the interface between the roller and pin might get up over 150F if the knife blade is getting up over 600. Not hot, not for long, but just enough for a tiny bit of capillary action to back fill the wax lost due to friction and pressure.
This effect is familiar to some knife sharpening people who are aware that dry honing has the effect of raising the temperature at the very edge momentarily high enough to affect the temper, leading to rolled edges. So I don't think it is far fetched to think that the interface between the roller and pin might get up over 150F if the knife blade is getting up over 600. Not hot, not for long, but just enough for a tiny bit of capillary action to back fill the wax lost due to friction and pressure.
Another take away from the article is an odd result they didn’t anticipate
In lab tests comparing the three products, there was no significant difference in energy efficiency. "Then we removed any lubricant from the chain and ran the test again," Spicer recalls. "We were surprised to find that the efficiency was essentially the same as when it was lubricated."
Speaking of which, do you really believe that wax is such a durable material that you can squeeze it between two hardened steel surfaces with the full weight of a rider standing on the crank - and it will last for 300 miles???? And we know it lasts, because dry chain pivots - waxed or not - squeak. But it takes hundreds of miles or lots of rain to squeak. Same thing that happens when the chain runs out of oil.
Wax is just an oil that is solid at room temperature and normal pressure. That's not the environment of the inside of a chain.
Wax is just an oil that is solid at room temperature and normal pressure. That's not the environment of the inside of a chain.
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Likes For cyccommute:
#102
Senior Member
Join Date: Apr 2011
Posts: 6,963
Mentioned: 41 Post(s)
Tagged: 0 Thread(s)
Quoted: 4342 Post(s)
Liked 1,528 Times
in
997 Posts
My thoughts on the heat generated are informed by this old article about a Johns Hopkins study on chain efficiency. They measured the heat generated (unfortunately the video links no longer work) and found very little heat generated by friction. They measured the efficiency at almost 99%. If the chain were heated, even momentarily, to the melting point of wax, it would show up.
Another take away from the article is an odd result they didn’t anticipate
That says to me that which lubricant used likely has little impact. I’m not brave enough to go without any kind of lubricant but it would make for an interesting experiment.
I’m not sure what you are trying to say here. Are you being facetious or serious? I have zero problem with the longevity of wax. As you know I use solvent wax and I get 600 to 700 miles out of an application. I’d expect similar out of hot wax. I don’t think the wax is melting due to pressure. It is “plastic” so it squeezes out of the pin/plate junction but doesn’t flow back like oil does. But, based on that Johns Hopkins article, lubrication probably isn’t all that important anyway.
Another take away from the article is an odd result they didn’t anticipate
That says to me that which lubricant used likely has little impact. I’m not brave enough to go without any kind of lubricant but it would make for an interesting experiment.
I’m not sure what you are trying to say here. Are you being facetious or serious? I have zero problem with the longevity of wax. As you know I use solvent wax and I get 600 to 700 miles out of an application. I’d expect similar out of hot wax. I don’t think the wax is melting due to pressure. It is “plastic” so it squeezes out of the pin/plate junction but doesn’t flow back like oil does. But, based on that Johns Hopkins article, lubrication probably isn’t all that important anyway.
#103
Senior Member
The strange thing is that softened waxes keep the chain quiet a long time but do not protect against wear as well as normal paraffin wax. Potentially it's because the soft wax gets squished out of metal contact but enough remains inside the chain in non contact areas to dampen noise.
Softened waxes like paraffin that has had oil or even another soft tacky wax added to it protect against corrosion poorly compared to paraffin wax. Testing against wear would require a more complicated setup than I have currently, but if the zero friction cycling person is to be believed, the wear charasteristics of softened waxes are really quite poor.
Likes For elcruxio:
#104
Senior Member
Join Date: Apr 2011
Posts: 6,963
Mentioned: 41 Post(s)
Tagged: 0 Thread(s)
Quoted: 4342 Post(s)
Liked 1,528 Times
in
997 Posts
Potentially a microfilm of wax in and on top of the porous structure of the steel. Thin enough not to get displaced easily by the limited movement a chain actually experiences but thick enough to protect against wear and/or corrosion. The chain starts making noise when steel is exposed and depending on wax that may take quite a while.
The strange thing is that softened waxes keep the chain quiet a long time but do not protect against wear as well as normal paraffin wax. Potentially it's because the soft wax gets squished out of metal contact but enough remains inside the chain in non contact areas to dampen noise.
Softened waxes like paraffin that has had oil or even another soft tacky wax added to it protect against corrosion poorly compared to paraffin wax. Testing against wear would require a more complicated setup than I have currently, but if the zero friction cycling person is to be believed, the wear charasteristics of softened waxes are really quite poor.
The strange thing is that softened waxes keep the chain quiet a long time but do not protect against wear as well as normal paraffin wax. Potentially it's because the soft wax gets squished out of metal contact but enough remains inside the chain in non contact areas to dampen noise.
Softened waxes like paraffin that has had oil or even another soft tacky wax added to it protect against corrosion poorly compared to paraffin wax. Testing against wear would require a more complicated setup than I have currently, but if the zero friction cycling person is to be believed, the wear charasteristics of softened waxes are really quite poor.
I'm not surprised that an unlubricated chain would be efficient. Hard wax mimics a dry chain as it goes through the pulleys under no load.
But It acts like a lubricant when under load - and I absolutely can't imagine that something as soft as wax is acting like bonded teflon for hundreds of miles at a time. That makes zero sense. As little as claiming the loads generated on pins can't possibly generate localized heat for very short periods.
#105
Randomhead
Join Date: Aug 2008
Location: Happy Valley, Pennsylvania
Posts: 24,387
Mentioned: 0 Post(s)
Tagged: 0 Thread(s)
Quoted: 4 Post(s)
Liked 3,687 Times
in
2,510 Posts
I'm curious if chain waxers have a whole case of quick links. Most of them are only rated for one re-use. The only chains I have ever broken were at the quick link. It's not a secret that I can use any watt I can scrounge, but this aspect makes me worry about waxing.
#106
Senior Member
Join Date: Jun 2008
Location: Louisville, KY
Posts: 13,443
Mentioned: 33 Post(s)
Tagged: 0 Thread(s)
Quoted: 4225 Post(s)
Liked 2,945 Times
in
1,804 Posts
I'm curious if chain waxers have a whole case of quick links. Most of them are only rated for one re-use. The only chains I have ever broken were at the quick link. It's not a secret that I can use any watt I can scrounge, but this aspect makes me worry about waxing.
Likes For himespau:
#107
Senior Member
I use connex links. They're beefy enough that they'll likely outlast chains easily. The only problem with them is that they do not as of yet have a 12-speed quick link, meaning that with 12-speed bikes I need to use what I can find. But even the single use ones are highly reusable.
#108
Just Pedaling
Join Date: Oct 2021
Location: US West Coast
Posts: 1,000
Bikes: YEP!
Mentioned: 0 Post(s)
Tagged: 0 Thread(s)
Quoted: 328 Post(s)
Liked 516 Times
in
343 Posts
I reuse the same quick link for the life of the chain. I click them on and off at least 2 dozen times before the chain goes into the recycle. Never a problem yet and I've been doing this for years. I average 6000 miles or 10000 kilometers/year using 2 chains in rotation. The 2 chains last almost 3 years doing this. That's @30 reuses/chain. The link gets waxed along with the chain. My experience. Your's may be different.
Likes For SpedFast:
#109
Senior Member
Join Date: Dec 2020
Location: Wake Forest, NC
Posts: 5,768
Bikes: 1989 Cinelli Supercorsa
Mentioned: 11 Post(s)
Tagged: 0 Thread(s)
Quoted: 3498 Post(s)
Liked 2,911 Times
in
1,766 Posts
As mentioned above, the Connex links are designed to be used over and over again. They don’t rely on a friction snap fit to wear out, and tools are unnecessary. Surprised they haven’t become an industry standard.
#110
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,342
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6201 Post(s)
Liked 4,204 Times
in
2,358 Posts
With oil, the oil gets pushed out of the contact point but it flows back as the pressure is released. It constantly refreshes. But all is not perfect with oil. Since the oil flows in and out of the contact point, it carries grit…especially extremely small grit…into the contact point. The result is a grinding paste that wears the chain out at about the same rate as the metal on metal contact so that it doesn’t make much difference which lubricant is used in terms of wear. There’s a big difference in terms of cleanliness, however.
If you have ever used mineral spirits to clean an oiled chain, you can see grit in the bottom of the bottle. There are even bits of steel in there too. If you’ve used mineral spirits to clean a waxed chain, you won’t see the grit but you will see some metal. There’s not as much because the wax squeezes out of the chain and spalls off, carrying the metal with it. Oil doesn’t lose as much material by this means because oil doesn’t really drip off.
Wiping the outside of a dirty oiled chain, by the way, results in pushing grit…especially that small grit…into the chain. One drip of oil on the outside of a dirty chain does something similar. Solvent wax is flooded through the chain so that it drips off the outside so as to flush out the (very) small amount of grit that might stick to the outside of the chain. It would probably be better to flood oil through the chain as well but then more wiping would be required. Hot waxing by its very nature is flooding the chain but, as with solvent wax, there isn’t much grit stuck to the outside of the chain.
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
#111
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,342
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6201 Post(s)
Liked 4,204 Times
in
2,358 Posts
Potentially a microfilm of wax in and on top of the porous structure of the steel. Thin enough not to get displaced easily by the limited movement a chain actually experiences but thick enough to protect against wear and/or corrosion. The chain starts making noise when steel is exposed and depending on wax that may take quite a while.
The strange thing is that softened waxes keep the chain quiet a long time but do not protect against wear as well as normal paraffin wax. Potentially it's because the soft wax gets squished out of metal contact but enough remains inside the chain in non contact areas to dampen noise.
Softened waxes like paraffin that has had oil or even another soft tacky wax added to it protect against corrosion poorly compared to paraffin wax. Testing against wear would require a more complicated setup than I have currently, but if the zero friction cycling person is to be believed, the wear charasteristics of softened waxes are really quite poor.
The strange thing is that softened waxes keep the chain quiet a long time but do not protect against wear as well as normal paraffin wax. Potentially it's because the soft wax gets squished out of metal contact but enough remains inside the chain in non contact areas to dampen noise.
Softened waxes like paraffin that has had oil or even another soft tacky wax added to it protect against corrosion poorly compared to paraffin wax. Testing against wear would require a more complicated setup than I have currently, but if the zero friction cycling person is to be believed, the wear charasteristics of softened waxes are really quite poor.
I take anything that Zero Friction says with a very large grain of salt. First, his cleaning procedures are unnecessarily complex. Second, his estimation of chain wear are wild overestimates based on what is commonly reported for chain wear. The soften wax used by chain manufacturers performs reasonably well in his tests…as far as I trust his test…when compared to other lubricants.
Part of the reason I’d suggest a soft wax has to do with the overly complicated cleaning procedure that Zero Friction says is needed. The reason for the multiple steps is to clean the chain so that the wax sticks to it. I really doubt that a systemic study has been done to see if all the cleaning steps are needed but a softer wax would stick to the metal better than hard wax will. (Note: I mean slightly softer, not straight up petrolatum, wax or a mixture of hard and soft waxes).
But It acts like a lubricant when under load - and I absolutely can't imagine that something as soft as wax is acting like bonded teflon for hundreds of miles at a time. That makes zero sense. As little as claiming the loads generated on pins can't possibly generate localized heat for very short periods.
As for heat generation, there just isn’t any. If you had enough heat to even locally melt wax, that heat will be absorbed into the metal of the chain and build over time. We are talking about a lot of heat to melt the wax even locally. The people at Johns Hopkins would have seen that heat grow over time which is not what they observed.
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
#112
Senior Member
Join Date: Apr 2011
Posts: 6,963
Mentioned: 41 Post(s)
Tagged: 0 Thread(s)
Quoted: 4342 Post(s)
Liked 1,528 Times
in
997 Posts
The wax squeezes out of the contact point but it really has no place to go since the rollers are full of wax. It squeezes out relatively slowly as more of the wax in the roller gets push all the way out of the chain. Eventually, it will completely starve the contact point, resulting in metal on metal contact. The chain wears out because of this metal on metal contact.
You get similar mileage to oil because it is an oil.
Last edited by Kontact; 02-15-23 at 12:26 PM.
#113
Senior Member
Join Date: Apr 2011
Posts: 6,963
Mentioned: 41 Post(s)
Tagged: 0 Thread(s)
Quoted: 4342 Post(s)
Liked 1,528 Times
in
997 Posts
The difference in how a hard wax and a soft wax react under pressure is going to be very small. The “hardness” of hard wax is still incredibly soft. It won’t stand up to much pressure before getting squished out of the contact point.
I take anything that Zero Friction says with a very large grain of salt. First, his cleaning procedures are unnecessarily complex. Second, his estimation of chain wear are wild overestimates based on what is commonly reported for chain wear. The soften wax used by chain manufacturers performs reasonably well in his tests…as far as I trust his test…when compared to other lubricants.
You really can’t talk about the surface tension of a solid. Surface tension is something that occurs in liquids as they try to minimize their volume. Solids can’t do that. Nonpolar compounds like oils have weak surface tension to begin with when compared to water or other polar substances. Again, I’m not clear on what you mean by “resist the chain pivoting”. Nothing that could be added to the chain will keep it from pivoting as it goes around the drivetrain.
Part of the reason I’d suggest a soft wax has to do with the overly complicated cleaning procedure that Zero Friction says is needed. The reason for the multiple steps is to clean the chain so that the wax sticks to it. I really doubt that a systemic study has been done to see if all the cleaning steps are needed but a softer wax would stick to the metal better than hard wax will. (Note: I mean slightly softer, not straight up petrolatum, wax or a mixture of hard and soft waxes).
I just do see why you think a softer wax wouldn’t act as a lubricant. The difference in hardness really isn’t a large as you are making it out to be, although the slightly harder wax is less flexible.
As for heat generation, there just isn’t any. If you had enough heat to even locally melt wax, that heat will be absorbed into the metal of the chain and build over time. We are talking about a lot of heat to melt the wax even locally. The people at Johns Hopkins would have seen that heat grow over time which is not what they observed.
I take anything that Zero Friction says with a very large grain of salt. First, his cleaning procedures are unnecessarily complex. Second, his estimation of chain wear are wild overestimates based on what is commonly reported for chain wear. The soften wax used by chain manufacturers performs reasonably well in his tests…as far as I trust his test…when compared to other lubricants.
You really can’t talk about the surface tension of a solid. Surface tension is something that occurs in liquids as they try to minimize their volume. Solids can’t do that. Nonpolar compounds like oils have weak surface tension to begin with when compared to water or other polar substances. Again, I’m not clear on what you mean by “resist the chain pivoting”. Nothing that could be added to the chain will keep it from pivoting as it goes around the drivetrain.
Part of the reason I’d suggest a soft wax has to do with the overly complicated cleaning procedure that Zero Friction says is needed. The reason for the multiple steps is to clean the chain so that the wax sticks to it. I really doubt that a systemic study has been done to see if all the cleaning steps are needed but a softer wax would stick to the metal better than hard wax will. (Note: I mean slightly softer, not straight up petrolatum, wax or a mixture of hard and soft waxes).
I just do see why you think a softer wax wouldn’t act as a lubricant. The difference in hardness really isn’t a large as you are making it out to be, although the slightly harder wax is less flexible.
As for heat generation, there just isn’t any. If you had enough heat to even locally melt wax, that heat will be absorbed into the metal of the chain and build over time. We are talking about a lot of heat to melt the wax even locally. The people at Johns Hopkins would have seen that heat grow over time which is not what they observed.
I didn't say a soft wax doesn't act as a lubricant. A lubricant is something that prevents wear on hard surfaces. That doesn't mean that a lubricant has to be efficient. This is one of the reasons oils are used in many places instead of grease. One only has to rub Tenacious Oil between your fingers to understand that clingy, stretchy and thick lubes absorb energy.
Your assertion that no heat on any level is generated in a mechanical system that is transimitting 300 watts is crazy talk. Of course there is some friction heat. The only question is if there is enough to momentarily liquify small amounts of wax or not.
#114
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,342
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6201 Post(s)
Liked 4,204 Times
in
2,358 Posts
I suspect that the tackiness of a soft wax would make it adhere to the metal surface better than a hard wax. It shears under load rather than fracture
I didn't say a soft wax doesn't act as a lubricant. A lubricant is something that prevents wear on hard surfaces. That doesn't mean that a lubricant has to be efficient. This is one of the reasons oils are used in many places instead of grease. One only has to rub Tenacious Oil between your fingers to understand that clingy, stretchy and thick lubes absorb energy.
Your assertion that no heat on any level is generated in a mechanical system that is transimitting 300 watts is crazy talk. Of course there is some friction heat. The only question is if there is enough to momentarily liquify small amounts of wax or not.
The wax certainly gets moved around…not as efficiently as oil…but it doesn’t liquify. There just not enough heat available to do that.
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
#115
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,342
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6201 Post(s)
Liked 4,204 Times
in
2,358 Posts
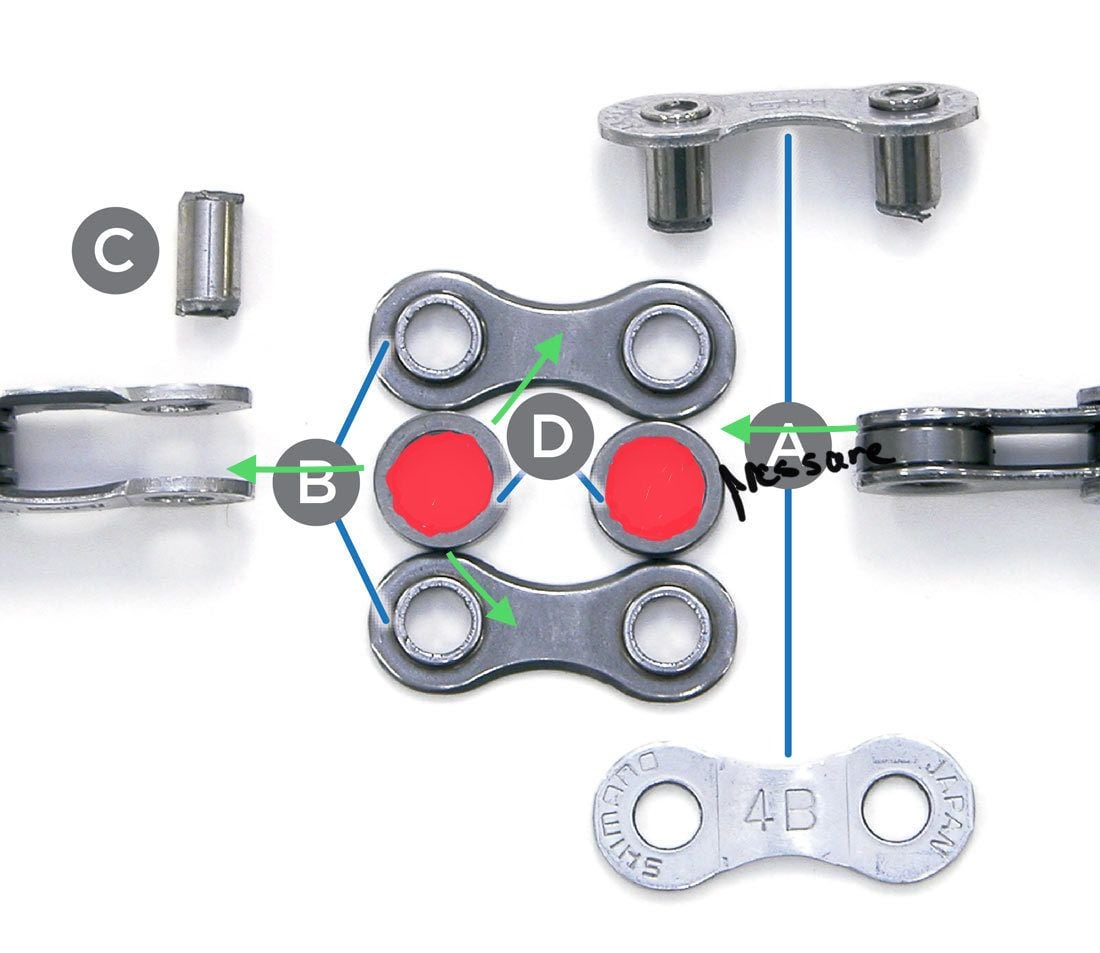
You also seem to be confused about what wax is doing inside the chain. With an assist from Park Tool let’s look at how oil and wax behave inside the chain.
When the chain is clean, the space in the rollers is empty as seen in the picture below.

When you put oil on the chain, it pools (blue in the picture) in the bottom of the rollers under the influence of gravity (yellow arrow) because it is a liquid and can flow. Pressure as you pedal is going to make a film of oil around the roller but the liquid is still mostly under the influence of gravity. The oil is going to run out of the chain primarily towards the bottom of the chain. When the chain isn’t moving, oil is going to pool again. The pressure at the contact point moves oil out of the contact point but it can flow back when pressure is released.

With wax, the roller is filled with a solid (red in the picture). It’s not a hard solid and barely above classification as a semi-solid but it is more solid than oil. Gravity has no influence on the material to make it flow. Pressure at the pins is going to push the wax into more wax. It really has no place to go. Release the pressure and the wax springs back into the void. Since the system isn’t sealed, some of the wax filing the space gets squeezed out of the plates and is lost to the world. It’s a little slower process than oil but eventually, enough wax gets squeezed out that the wax is pushing into a bit of a void and can’t spring back. You eventually end up with the pressure point being starved of lubrication and it needs to be refreshed.
The void and lubrication starvation is the cause of the problem with wax and rain. I will state, unequivocally, that rain cannot wash off wax. Wax has zero water solubility…even a soft wax like petroleum jelly. When riding in rain on a waxed chain, the void created by pressure allows water to sit on the metal on metal surface created by (mostly) outward movement of wax. This leads to oxidation of the chain and the characteristic “squeak” of a waxed chain after rain. The wax isn’t washed off (because it can’t be) but there is metal exposure and subsequent rusting when water is introduced.
The rain problem with wax also argues against your “chain pressure melting wax” idea. If there were enough heat to melt the wax and make it flow back into the contact points, rain would never be a problem. Melted wax would fill back into space to make exposed metal, and thus the rain induced squeak, impossible.
Further, a softer wax mixture would facilitate the “spring back” since it is more flexible.
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Likes For cyccommute:
#116
Garage tetris expert
Join Date: May 2016
Location: Texas Hill Country
Posts: 889
Bikes: A few. Ok, a lot
Mentioned: 16 Post(s)
Tagged: 0 Thread(s)
Quoted: 386 Post(s)
Liked 691 Times
in
328 Posts
Really fantastic explanation @cyccommute, thanks!
#118
Senior Member
Join Date: Apr 2011
Posts: 6,963
Mentioned: 41 Post(s)
Tagged: 0 Thread(s)
Quoted: 4342 Post(s)
Liked 1,528 Times
in
997 Posts
You absolutely did say that soft wax isn’t a lubricant. The amount of energy absorbed is also very small when it comes to bicycle chains. The 2013 Velonews/Friction Facts article compares paraffin to Tenacious Oil. the difference between them is 1.3 W (4.8W to 6.1W). And, again, we have no idea what the margin of error on the measurement is. If the error is 0.1W, that could be significant. But if the error is 2W, no conclusion can be drawn. Without the error measurement, we can’t say if it’s as large a difference as presented.
But what was being discussed is that lubricants are there to prevent metal on metal wear. Wax does that for long periods, which is why the chain doesn't squeak. Wax is a low drag lubricant - probably because it is mostly solid and completely untacky, yet still provides sufficient lubrication where the force is applied. Efficient lubricants are those that prevent wear with a minimum of their own drag.
Yes, the drivetrain is transmitting 300W but, according to the Johns Hopkins study, 99.5% of that, or 298.5W, is being translated to the ground. 1.5W is being wasted as heat. Since the chain is not adiabatic, the heat is lost constantly. And, since the chain is under constant airflow, that heat would be quickly stripped away. Basically, there is no way that any part of the chain could heat to the 150°F needed to melt wax…even locally. Steel is very good at heat transmission.
The wax certainly gets moved around…not as efficiently as oil…but it doesn’t liquify. There just not enough heat available to do that.
The wax certainly gets moved around…not as efficiently as oil…but it doesn’t liquify. There just not enough heat available to do that.
Your arguments are very confusing. Are you saying that wax doesn’t last while also arguing that wax provides lasting protection against friction? Are you saying that wax has to be refreshed every 20 hours (every 200 miles or so)? You’ve also said that wax isn’t oil but now it is an oil?
You also seem to be confused about what wax is doing inside the chain. With an assist from Park Tool let’s look at how oil and wax behave inside the chain.
You also seem to be confused about what wax is doing inside the chain. With an assist from Park Tool let’s look at how oil and wax behave inside the chain.
I know what a chain looks like. The points I've been referring to are where the pins run through the inner links. The surface area is so small and the pressure so great that there is NO WAY solid wax forms a barrier that lasts for 200 hours or more under load.
The void and lubrication starvation is the cause of the problem with wax and rain. I will state, unequivocally, that rain cannot wash off wax. Wax has zero water solubility…even a soft wax like petroleum jelly. When riding in rain on a waxed chain, the void created by pressure allows water to sit on the metal on metal surface created by (mostly) outward movement of wax. This leads to oxidation of the chain and the characteristic “squeak” of a waxed chain after rain. The wax isn’t washed off (because it can’t be) but there is metal exposure and subsequent rusting when water is introduced.
The rain problem with wax also argues against your “chain pressure melting wax” idea. If there were enough heat to melt the wax and make it flow back into the contact points, rain would never be a problem. Melted wax would fill back into space to make exposed metal, and thus the rain induced squeak, impossible.
The rain problem with wax also argues against your “chain pressure melting wax” idea. If there were enough heat to melt the wax and make it flow back into the contact points, rain would never be a problem. Melted wax would fill back into space to make exposed metal, and thus the rain induced squeak, impossible.
But rain definitely shortens the life of a waxing. Why? Because water washes out the wax when it liquifies. Since you don't ride immersion waxed chains in a rainy climate, you wouldn't know anything about what you're claiming.
I would address your assertions that gravity is a bigger force than surface tension for oils, but why bother?
#119
Senior Member
I just can't see how even limited internal partitions of the chain could heat up enough to melt sufficient amounts of wax long enough to create an oil like lubricant inside the chain.
Heating 100 milligrams of steel by 50 degrees celsius in one second takes more than 2 watts. Even with power of 300 watts you'd transform only around 1,5 watts to heat in the chain. Not all of it is going to go to the correct place for relevant wax melting. That 100 milligrams is 0,04 % of the mass of a 114 link chain. Each link is roughly 2 grams in weight. That 100 milligrams would only by 5% of the mass of one link.
What I'm getting at that the amount of chain you can heat with 2 - 3 watts to wax melting temperatures is so small, that it's inplausible that you'd be able to melt enough wax for lubrication or redistribution.
If the heating happens in the wax itself via the deformation the chain introduces by pressure, that just means the wax won't redistribute. The layer of wax will just get thinner by every deformation as the wax won't have time to flow back after the pressure of the opposing steel pieces relaxes. The steel itself will suck out all of the heat of the melted wax
Even if you could add enough heat to the correct places inside the chain, you'll have conductive heat transfer from the affected frinction receiving surfaces to the other parts of the chain. You'll have airflow cooling the chain. Even though steel isn't the best heat conductor, it's still a metal and as such pretty darn conductive. AND you'll need to heat the wax involved, which'll require a temperature that's somewhat higher than the melting point of the wax in order to melt enough of it fast enough for it to distribute. The period where a chain link experiences enough pressure for sufficient heat to occur is pretty short after which it'll inevitably start cooling off.
I also find it highly unlikely that water would be able to flush off heated liquid wax from inside the chain. Any water that makes contact with liquid wax is going to cool down that wax really quickly. Perhaps the wax can then flake off (unlikely. It's really hard to boil a wax pot clean. That stuff sticks like crazy), but then it's still stuck inside the chain.
Additionally wax wouldn't work in the dead of winter. If you suddenly have to add 90 degrees celsius of heat instead of 50, the wattage required gets pretty ridiculous. And in my experience waxed chains work best and last longest in sub freezing dry cold air. Last winter I waxed my fatbike chain once. I only rode it in sub freezing.
To top it all off, that 2 -3 watts energy loss for the chain occurs at 300 watts. That is a lot of power for even seasoned amateurs. But what if you're cycling only with 50 watts? The wax still works even at those low wattages when there's only 0.5 watts available for the wax melting.
Can't happen. There's not enough energy available.
Heating 100 milligrams of steel by 50 degrees celsius in one second takes more than 2 watts. Even with power of 300 watts you'd transform only around 1,5 watts to heat in the chain. Not all of it is going to go to the correct place for relevant wax melting. That 100 milligrams is 0,04 % of the mass of a 114 link chain. Each link is roughly 2 grams in weight. That 100 milligrams would only by 5% of the mass of one link.
What I'm getting at that the amount of chain you can heat with 2 - 3 watts to wax melting temperatures is so small, that it's inplausible that you'd be able to melt enough wax for lubrication or redistribution.
If the heating happens in the wax itself via the deformation the chain introduces by pressure, that just means the wax won't redistribute. The layer of wax will just get thinner by every deformation as the wax won't have time to flow back after the pressure of the opposing steel pieces relaxes. The steel itself will suck out all of the heat of the melted wax
Even if you could add enough heat to the correct places inside the chain, you'll have conductive heat transfer from the affected frinction receiving surfaces to the other parts of the chain. You'll have airflow cooling the chain. Even though steel isn't the best heat conductor, it's still a metal and as such pretty darn conductive. AND you'll need to heat the wax involved, which'll require a temperature that's somewhat higher than the melting point of the wax in order to melt enough of it fast enough for it to distribute. The period where a chain link experiences enough pressure for sufficient heat to occur is pretty short after which it'll inevitably start cooling off.
I also find it highly unlikely that water would be able to flush off heated liquid wax from inside the chain. Any water that makes contact with liquid wax is going to cool down that wax really quickly. Perhaps the wax can then flake off (unlikely. It's really hard to boil a wax pot clean. That stuff sticks like crazy), but then it's still stuck inside the chain.
Additionally wax wouldn't work in the dead of winter. If you suddenly have to add 90 degrees celsius of heat instead of 50, the wattage required gets pretty ridiculous. And in my experience waxed chains work best and last longest in sub freezing dry cold air. Last winter I waxed my fatbike chain once. I only rode it in sub freezing.
To top it all off, that 2 -3 watts energy loss for the chain occurs at 300 watts. That is a lot of power for even seasoned amateurs. But what if you're cycling only with 50 watts? The wax still works even at those low wattages when there's only 0.5 watts available for the wax melting.
Can't happen. There's not enough energy available.
Likes For elcruxio:
#120
Senior Member
Join Date: Apr 2011
Posts: 6,963
Mentioned: 41 Post(s)
Tagged: 0 Thread(s)
Quoted: 4342 Post(s)
Liked 1,528 Times
in
997 Posts
I just can't see how even limited internal partitions of the chain could heat up enough to melt sufficient amounts of wax long enough to create an oil like lubricant inside the chain.
Heating 100 milligrams of steel by 50 degrees celsius in one second takes more than 2 watts. Even with power of 300 watts you'd transform only around 1,5 watts to heat in the chain. Not all of it is going to go to the correct place for relevant wax melting. That 100 milligrams is 0,04 % of the mass of a 114 link chain. Each link is roughly 2 grams in weight. That 100 milligrams would only by 5% of the mass of one link.
What I'm getting at that the amount of chain you can heat with 2 - 3 watts to wax melting temperatures is so small, that it's inplausible that you'd be able to melt enough wax for lubrication or redistribution.
If the heating happens in the wax itself via the deformation the chain introduces by pressure, that just means the wax won't redistribute. The layer of wax will just get thinner by every deformation as the wax won't have time to flow back after the pressure of the opposing steel pieces relaxes. The steel itself will suck out all of the heat of the melted wax
Even if you could add enough heat to the correct places inside the chain, you'll have conductive heat transfer from the affected frinction receiving surfaces to the other parts of the chain. You'll have airflow cooling the chain. Even though steel isn't the best heat conductor, it's still a metal and as such pretty darn conductive. AND you'll need to heat the wax involved, which'll require a temperature that's somewhat higher than the melting point of the wax in order to melt enough of it fast enough for it to distribute. The period where a chain link experiences enough pressure for sufficient heat to occur is pretty short after which it'll inevitably start cooling off.
I also find it highly unlikely that water would be able to flush off heated liquid wax from inside the chain. Any water that makes contact with liquid wax is going to cool down that wax really quickly. Perhaps the wax can then flake off (unlikely. It's really hard to boil a wax pot clean. That stuff sticks like crazy), but then it's still stuck inside the chain.
Additionally wax wouldn't work in the dead of winter. If you suddenly have to add 90 degrees celsius of heat instead of 50, the wattage required gets pretty ridiculous. And in my experience waxed chains work best and last longest in sub freezing dry cold air. Last winter I waxed my fatbike chain once. I only rode it in sub freezing.
To top it all off, that 2 -3 watts energy loss for the chain occurs at 300 watts. That is a lot of power for even seasoned amateurs. But what if you're cycling only with 50 watts? The wax still works even at those low wattages when there's only 0.5 watts available for the wax melting.
Can't happen. There's not enough energy available.
Heating 100 milligrams of steel by 50 degrees celsius in one second takes more than 2 watts. Even with power of 300 watts you'd transform only around 1,5 watts to heat in the chain. Not all of it is going to go to the correct place for relevant wax melting. That 100 milligrams is 0,04 % of the mass of a 114 link chain. Each link is roughly 2 grams in weight. That 100 milligrams would only by 5% of the mass of one link.
What I'm getting at that the amount of chain you can heat with 2 - 3 watts to wax melting temperatures is so small, that it's inplausible that you'd be able to melt enough wax for lubrication or redistribution.
If the heating happens in the wax itself via the deformation the chain introduces by pressure, that just means the wax won't redistribute. The layer of wax will just get thinner by every deformation as the wax won't have time to flow back after the pressure of the opposing steel pieces relaxes. The steel itself will suck out all of the heat of the melted wax
Even if you could add enough heat to the correct places inside the chain, you'll have conductive heat transfer from the affected frinction receiving surfaces to the other parts of the chain. You'll have airflow cooling the chain. Even though steel isn't the best heat conductor, it's still a metal and as such pretty darn conductive. AND you'll need to heat the wax involved, which'll require a temperature that's somewhat higher than the melting point of the wax in order to melt enough of it fast enough for it to distribute. The period where a chain link experiences enough pressure for sufficient heat to occur is pretty short after which it'll inevitably start cooling off.
I also find it highly unlikely that water would be able to flush off heated liquid wax from inside the chain. Any water that makes contact with liquid wax is going to cool down that wax really quickly. Perhaps the wax can then flake off (unlikely. It's really hard to boil a wax pot clean. That stuff sticks like crazy), but then it's still stuck inside the chain.
Additionally wax wouldn't work in the dead of winter. If you suddenly have to add 90 degrees celsius of heat instead of 50, the wattage required gets pretty ridiculous. And in my experience waxed chains work best and last longest in sub freezing dry cold air. Last winter I waxed my fatbike chain once. I only rode it in sub freezing.
To top it all off, that 2 -3 watts energy loss for the chain occurs at 300 watts. That is a lot of power for even seasoned amateurs. But what if you're cycling only with 50 watts? The wax still works even at those low wattages when there's only 0.5 watts available for the wax melting.
Can't happen. There's not enough energy available.
You don't need to believe me that is exactly what happens. You just need to ask yourself if a coating of wax thin enough to separate two very small pieces of steel, bearing all your body weight, is going to last for 300 miles. It's only wax, not some sort of industrial molybdenum sulfide or teflon coating. Meanwhile, the forces on the chain are sufficient to wear the inside of the rollers out, even on a well lubricated chain.
Does not compute. Hard wax is neither super grippy nor super lubricating nor super tough.
#121
Senior Member
You don't need to believe me that is exactly what happens. You just need to ask yourself if a coating of wax thin enough to separate two very small pieces of steel, bearing all your body weight, is going to last for 300 miles. It's only wax, not some sort of industrial molybdenum sulfide or teflon coating. Meanwhile, the forces on the chain are sufficient to wear the inside of the rollers out, even on a well lubricated chain.
The second problem is the limitation of oil as a bushing lubricant. Kinda. A bicycle chain doesn't experience enough speed or the correct movement directions to isolate the metal pieces from making contact with one another. So there's constant metal to metal wear.
A grease with a better staying power and squish resistance would work better. It better isolates the metal parts from one another, ie. makes them float against one another. Grease could potentially prevent wear significantly but getting it inside the chain is difficult and it can't be used in the wild as it'll attract everything smaller than a squirrel. Also it'll squeeze out eventually leaving a film too thin to prevent metal on metal contact.
A wax on the other hand does not attract anything. It'll isolate the metal pieces by being too solid to easily squeeze out and by creating a thin film adhering to the metal. First the thicker wax inside the chain gets sheared off but has no place to go. It'll get pushed out after it's ground down to small enough stuff to squeeze between the gaps. That'll take some time. All the while the inside of the chain is protected by the remaining wax film. Now how well that wax film works after the excess wax has been pushed out is debatable. Could be for fairly long or no time at all. My guess is that a harder wax stays for quite a while as wax can last for quite a long time against abrasion. Think ski wax. That stuff can last grinding against icy snow for a looong time. And inside the chain it's wax against wax until metal starts showing.
Does not compute. Hard wax is neither super grippy nor super lubricating nor super tough.
#122
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,342
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6201 Post(s)
Liked 4,204 Times
in
2,358 Posts
But what was being discussed is that lubricants are there to prevent metal on metal wear. Wax does that for long periods, which is why the chain doesn't squeak. Wax is a low drag lubricant - probably because it is mostly solid and completely untacky, yet still provides sufficient lubrication where the force is applied. Efficient lubricants are those that prevent wear with a minimum of their own drag.
Again, there is heat produced - as you admit. That heat doesn't have to get the whole chain to increase in temperature for a very confined area to get briefly warm. And there are two spots on the chain that pivot under load which could account for the majority of that heat. Just as you can hold a pipe that is being tig welded on the other end, a piece of metal doesn't have to heat evenly.
I know what a chain looks like. The points I've been referring to are where the pins run through the inner links. The surface area is so small and the pressure so great that there is NO WAY solid wax forms a barrier that lasts for 200 hours or more under load.
Rain has nothing to do with anything. I never mentioned rain at all. Waxed chains ridden in dry, cool conditions lose pivot wax until they squeak. According to you this can't be happening.
Think about what you are suggesting for a minute. The wax gets pushed out of the pin/plate interface when the chain goes over the gears. A small amount of wax gets melted and flows back into the space. The amount of wax needed would be on the order of micrograms and you are likely to have, perhaps a gram of wax available. That’s a million times what is needed so it should never go away.
Just to be clear, that’s your scenario, not mine.
But rain definitely shortens the life of a waxing. Why? Because water washes out the wax when it liquifies. Since you don't ride immersion waxed chains in a rainy climate, you wouldn't know anything about what you're claiming.
Now on to wax and water. Have you ever dripped wax onto water? It solidifies almost immediately. And the thinner the layer of wax, the faster it is going to solidify. You might be able to get wax to melt if the water is over the melting point of the wax but that’s not a water temperature you are likely to experience while riding a bicycle. If…and that is a very, very, very large “if”…the wax melted at all on the chain while riding, water is going to cool that wax and metal quickly below the melt point and solidify the wax. The wax wouldn’t wash off in any way. Wax won’t wash off of anything in water. Even if the water is over 150°F, it is difficult to get wax to melt and move.
By the way, oil is far more likely to float off a surface when exposed to water than wax ever would. I have long advocated that just because wax squeaks after rain and needs to be reapplied, oil doesn’t perform any better and the liquid nature of oil just masks the oxidation problem rather than solve it. Oil should be refreshed after just as wax does.
I would address your assertions that gravity is a bigger force than surface tension for oils, but why bother?
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Last edited by cyccommute; 02-16-23 at 10:47 AM.
#123
Mad bike riding scientist
Join Date: Nov 2004
Location: Denver, CO
Posts: 27,342
Bikes: Some silver ones, a red one, a black and orange one, and a few titanium ones
Mentioned: 152 Post(s)
Tagged: 1 Thread(s)
Quoted: 6201 Post(s)
Liked 4,204 Times
in
2,358 Posts
Well the problem with oil is twofold. Firstly even if you run the bike in a clean room, the oil inside the chain is going to get contaminated because of metal to metal contact. Additional grit quickens the effect.
The second problem is the limitation of oil as a bushing lubricant. Kinda. A bicycle chain doesn't experience enough speed or the correct movement directions to isolate the metal pieces from making contact with one another. So there's constant metal to metal wear.
A grease with a better staying power and squish resistance would work better. It better isolates the metal parts from one another, ie. makes them float against one another. Grease could potentially prevent wear significantly but getting it inside the chain is difficult and it can't be used in the wild as it'll attract everything smaller than a squirrel. Also it'll squeeze out eventually leaving a film too thin to prevent metal on metal contact.
A wax on the other hand does not attract anything. It'll isolate the metal pieces by being too solid to easily squeeze out and by creating a thin film adhering to the metal. First the thicker wax inside the chain gets sheared off but has no place to go. It'll get pushed out after it's ground down to small enough stuff to squeeze between the gaps. That'll take some time. All the while the inside of the chain is protected by the remaining wax film. Now how well that wax film works after the excess wax has been pushed out is debatable. Could be for fairly long or no time at all. My guess is that a harder wax stays for quite a while as wax can last for quite a long time against abrasion. Think ski wax. That stuff can last grinding against icy snow for a looong time. And inside the chain it's wax against wax until metal starts showing.
A grease with a better staying power and squish resistance would work better. It better isolates the metal parts from one another, ie. makes them float against one another. Grease could potentially prevent wear significantly but getting it inside the chain is difficult and it can't be used in the wild as it'll attract everything smaller than a squirrel. Also it'll squeeze out eventually leaving a film too thin to prevent metal on metal contact.
A wax on the other hand does not attract anything. It'll isolate the metal pieces by being too solid to easily squeeze out and by creating a thin film adhering to the metal. First the thicker wax inside the chain gets sheared off but has no place to go. It'll get pushed out after it's ground down to small enough stuff to squeeze between the gaps. That'll take some time. All the while the inside of the chain is protected by the remaining wax film. Now how well that wax film works after the excess wax has been pushed out is debatable. Could be for fairly long or no time at all. My guess is that a harder wax stays for quite a while as wax can last for quite a long time against abrasion. Think ski wax. That stuff can last grinding against icy snow for a looong time. And inside the chain it's wax against wax until metal starts showing.
Wax, on the other hand, fills the voids in the chain and doesn’t move. There is no mechanism for moving grit into the system so that it can grind on the metal.
Depending on the formulation, hard wax can be really adherent to metal. It isn't super lubricating but it doesn't need to be. It's surprisingly tough but that might not be the deciding factor.
__________________
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
Stuart Black
Plan Epsilon Around Lake Michigan in the era of Covid
Old School…When It Wasn’t Ancient bikepacking
Gold Fever Three days of dirt in Colorado
Pokin' around the Poconos A cold ride around Lake Erie
Dinosaurs in Colorado A mountain bike guide to the Purgatory Canyon dinosaur trackway
Solo Without Pie. The search for pie in the Midwest.
Picking the Scablands. Washington and Oregon, 2005. Pie and spiders on the Columbia River!
#124
Senior Member
Join Date: Apr 2011
Posts: 6,963
Mentioned: 41 Post(s)
Tagged: 0 Thread(s)
Quoted: 4342 Post(s)
Liked 1,528 Times
in
997 Posts
Okay, maybe I’ll grant you that. You said that certain waxes and oils weren’t good lubricants. You are wrong but anyway…
Chains are polished metal-on-metal surfaces under relatively light loads. The only reason they squeak is because the polished surface becomes less polished due to oxidation. Drag of the lubricant really has very little to do with a lubricant’s efficiency, especially under such light loads as bicycles put on the chain. The maximum load that is put on the bicycle chain is the weight of the rider. That’s also the maximum load that is put on the metal on metal contact.
I’ll admit to a very, very small amount of heat produced by friction. 0.5% of whatever is put into the chain is lost to heat. As elcruxio has pointed out, they’re just not enough heat available…even highly localized…to do what you want it to do. And, yes, you can have localized heating of a piece of metal but that heating works in both directions. Heat put into one place on a piece of metal is quickly distributed to the part of the metal that is u.n. heated and the overall temperature of the metal rises. Heat put into one end of a piece of metal is going to eventually end up in the other end. To torture this metaphor further, if you are to apply the heat of that weld to only one end of a piece of metal for a very long time, the whole bar will eventually be hot even with radiative cooling. A better analogy using welding would be a series of welds moving up the piece of metal. The whole piece is going to quickly get hot. At 90 rpm of the cranks, the each link of the chain is passing the point of highest pressure 90 times a minute. If you had sufficient heat to melt the wax, you could feel it at the end of the ride. Radiative cooling happens but not that quickly.
The pressure really isn’t all that great. The wax inside the rollers is somewhat elastic and the void is filled. You don’t need, nor have, much wax on the pins to provide lubrication. Under even the light pressure of a bicycle chain, I’d suspect that the layer of lubrication is, at best, microns thick. You wouldn’t need much wax touching the pin to drag some back into the space where it has been pushed out of.
You completely missed the point on rain. If the wax were melting like you suggest, the wax should fill back into the void and should keep the chain from squeaking after rain and reapplication after rain wouldn’t be necessary. In fact, given the amount of wax that is held up in the rollers of a chain, if wax melted like you suggest, you would likely never need to rewax a chain.
Think about what you are suggesting for a minute. The wax gets pushed out of the pin/plate interface when the chain goes over the gears. A small amount of wax gets melted and flows back into the space. The amount of wax needed would be on the order of micrograms and you are likely to have, perhaps a gram of wax available. That’s a million times what is needed so it should never go away.
Just to be clear, that’s your scenario, not mine.
First, don’t pull that “you don’t ride where I ride” crap. I’m not unfamiliar with rain riding. I don’t just ride when it is dry nor do I ride where it never rains. Solution waxing uses the same ideas as immersion waxing does with similar results and similar pitfalls…no matter what you may think. Second, I spent a significant amount of my life studying and working with chemicals. I know a whole lot about their properties and how they act and what they do.
Now on to wax and water. Have you ever dripped wax onto water? It solidifies almost immediately. And the thinner the layer of wax, the faster it is going to solidify. You might be able to get wax to melt if the water is over the melting point of the wax but that’s not a water temperature you are likely to experience while riding a bicycle. If…and that is a very, very, very large “if”…the wax melted at all on the chain while riding, water is going to cool that wax and metal quickly below the melt point and solidify the wax. The wax wouldn’t wash off in any way. Wax won’t wash off of anything in water. Even if the water is over 150°F, it is difficult to get wax to melt and move.
By the way, oil is far more likely to float off a surface when exposed to water than wax ever would. I have long advocated that just because wax squeaks after rain and needs to be reapplied, oil doesn’t perform any better and the liquid nature of oil just masks the oxidation problem rather than solve it. Oil should be refreshed after just as wax does.
Oh, by all means, explain surface tension of nonpolar compounds to me. I need something to chuckle at. For that matter, how about explaining to me how surface tension of a polar substance like water is greater than gravity while you are at it.
Chains are polished metal-on-metal surfaces under relatively light loads. The only reason they squeak is because the polished surface becomes less polished due to oxidation. Drag of the lubricant really has very little to do with a lubricant’s efficiency, especially under such light loads as bicycles put on the chain. The maximum load that is put on the bicycle chain is the weight of the rider. That’s also the maximum load that is put on the metal on metal contact.
I’ll admit to a very, very small amount of heat produced by friction. 0.5% of whatever is put into the chain is lost to heat. As elcruxio has pointed out, they’re just not enough heat available…even highly localized…to do what you want it to do. And, yes, you can have localized heating of a piece of metal but that heating works in both directions. Heat put into one place on a piece of metal is quickly distributed to the part of the metal that is u.n. heated and the overall temperature of the metal rises. Heat put into one end of a piece of metal is going to eventually end up in the other end. To torture this metaphor further, if you are to apply the heat of that weld to only one end of a piece of metal for a very long time, the whole bar will eventually be hot even with radiative cooling. A better analogy using welding would be a series of welds moving up the piece of metal. The whole piece is going to quickly get hot. At 90 rpm of the cranks, the each link of the chain is passing the point of highest pressure 90 times a minute. If you had sufficient heat to melt the wax, you could feel it at the end of the ride. Radiative cooling happens but not that quickly.
The pressure really isn’t all that great. The wax inside the rollers is somewhat elastic and the void is filled. You don’t need, nor have, much wax on the pins to provide lubrication. Under even the light pressure of a bicycle chain, I’d suspect that the layer of lubrication is, at best, microns thick. You wouldn’t need much wax touching the pin to drag some back into the space where it has been pushed out of.
You completely missed the point on rain. If the wax were melting like you suggest, the wax should fill back into the void and should keep the chain from squeaking after rain and reapplication after rain wouldn’t be necessary. In fact, given the amount of wax that is held up in the rollers of a chain, if wax melted like you suggest, you would likely never need to rewax a chain.
Think about what you are suggesting for a minute. The wax gets pushed out of the pin/plate interface when the chain goes over the gears. A small amount of wax gets melted and flows back into the space. The amount of wax needed would be on the order of micrograms and you are likely to have, perhaps a gram of wax available. That’s a million times what is needed so it should never go away.
Just to be clear, that’s your scenario, not mine.
First, don’t pull that “you don’t ride where I ride” crap. I’m not unfamiliar with rain riding. I don’t just ride when it is dry nor do I ride where it never rains. Solution waxing uses the same ideas as immersion waxing does with similar results and similar pitfalls…no matter what you may think. Second, I spent a significant amount of my life studying and working with chemicals. I know a whole lot about their properties and how they act and what they do.
Now on to wax and water. Have you ever dripped wax onto water? It solidifies almost immediately. And the thinner the layer of wax, the faster it is going to solidify. You might be able to get wax to melt if the water is over the melting point of the wax but that’s not a water temperature you are likely to experience while riding a bicycle. If…and that is a very, very, very large “if”…the wax melted at all on the chain while riding, water is going to cool that wax and metal quickly below the melt point and solidify the wax. The wax wouldn’t wash off in any way. Wax won’t wash off of anything in water. Even if the water is over 150°F, it is difficult to get wax to melt and move.
By the way, oil is far more likely to float off a surface when exposed to water than wax ever would. I have long advocated that just because wax squeaks after rain and needs to be reapplied, oil doesn’t perform any better and the liquid nature of oil just masks the oxidation problem rather than solve it. Oil should be refreshed after just as wax does.
Oh, by all means, explain surface tension of nonpolar compounds to me. I need something to chuckle at. For that matter, how about explaining to me how surface tension of a polar substance like water is greater than gravity while you are at it.
And heat isn't going to build in a chain that is using the heat to change the state of the wax, is radiating into the rings and cogs and is cooling in the air. Thats a silly argument that the heat has nowhere to go.
Last edited by Kontact; 02-16-23 at 11:45 AM.
#125
Senior Member
Serving as a bit of a translator here, you, of course, mean 50°C. Of course you are correct that it takes a lot of energy to get a mass to that temperature. It’s even worse since wax has a range of melt points up to 70°C (155°F). Even highly localized heating from friction is going to leave enough residual heat around that the overall temperature of the chain would increase over time. In other words, the chain would be at least warm to the touch at the end of a ride which isn’t something that happens.
To be fair, the same metal-on-metal wear happens with wax…either immersion or solvent. Waxes will turn gray over time which is the finely divided metal from that wear.
All good points. I would say that grit gets deposited on the outside of the chain just like with oil. I would posit that just as much grit per mile gets deposited on the chain with either lubricant. The oil, however, flows into and out of the chain as it gets moved around the drivetrain. The grit gets pumped into the chain where it does its damage. The only grit that matters is the grit that is harder than the steel which is most likely quartz because there is a ton of quartz particles on just about every square inch of earth.
All good points. I would say that grit gets deposited on the outside of the chain just like with oil. I would posit that just as much grit per mile gets deposited on the chain with either lubricant. The oil, however, flows into and out of the chain as it gets moved around the drivetrain. The grit gets pumped into the chain where it does its damage. The only grit that matters is the grit that is harder than the steel which is most likely quartz because there is a ton of quartz particles on just about every square inch of earth.
To go back to my above comment about metal-on-metal wear occurring with wax, while wax is good at avoiding the grit problem of oil…and the inherent messiness of oil…it does have significant metal-on-metal wear that contribute to wax not being all that much better as a lubricant than oil. About the only place where it is superior is in cleanliness. However, given the choice between cleaning up oil and somewhat frequent waxing, I’d go with wax every time.
I know you don't put much faith in the zero friction guy, but if his testing is as legit as it seems, judging from his findings, waxing with enough frequency could make for an almost eternal chain. It would seem that in the first 200km there's practically no wear with MSwax or Silca wax. Or paraffin for that matter.
Having discussed with someone who's product has been tested and is being tested presently by zero friction, the testing protocol and conclusions seem to be credible.
Of course waxing every 200km for a fair weather road bike is a bit too frequent as that's one or two rides. Which is why I started making my own stuff. 200km in bad weather is already something to write home about.